At surface of a liquid will their will be no forces (forces by molecules) acting on molecules in opposite directions such that the net force between molecules (only at surface) will be zero? If exists how surface area of a liquid contracts?
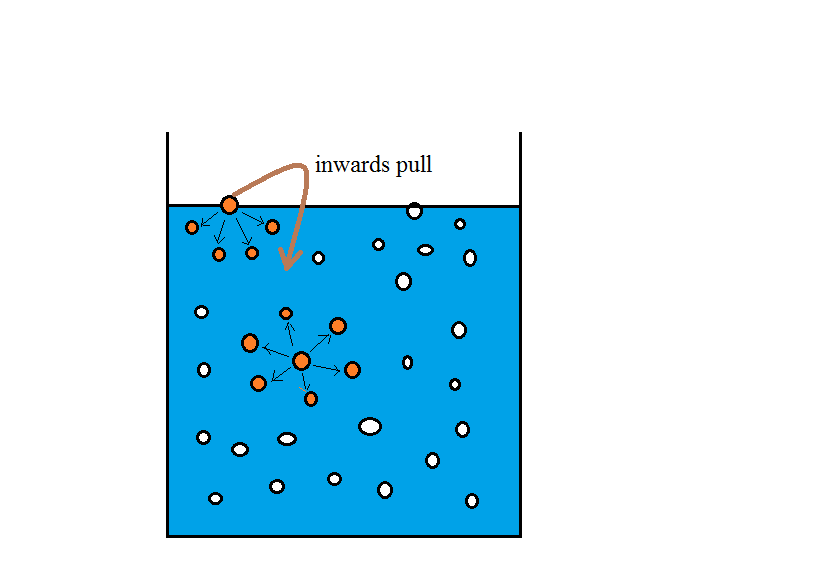
In the light of above concept let me explain a simple thing that is the inter-molecular attractions are there in between the particles. In case of liquids the particles are free to move in bulk as the particle is equally attracted from all sides. but in case of particles present on the surface, they are attracted from the is the particle is attracted inwards. And thus there is a tension on it also called surface tension.

It is evident from the above diagram that the particle near the surface is having a inward pull due to the attraction of inner particles.
but on the other hand the particle shown in the depth is getting attracted from all the sides thus all forces nullify each other and particle do not feel any attraction or tension.
Leave a Reply